Fernando Botto –
A general rule dictates “the more the antithombotic effect, the higher the risk of bleeding”.
In stable CAD patients beyond 1 year of PCI or without prior PCI with AF receiving OAC, clinical guidelines (ESC, Canadian) have suggested stopping ASA to reduce the risk of a major bleeding without a significant change in the anti-ischemic effect, based on observational studies and indirect evidence emerged from RCTs performed in stable CAD patients with or without AF. During the 2019 ESC Congress a new trial was presented.
Research question
In patients with AF and stable CAD (≥1 year after coronary revascularization or not suitable for revascularization) will Rivaroxaban monotherapy be non-inferior to a single antiplatelet agent (i.e. aspirin) plus Rivaroxaban in regards to a composite of cardiovascular events and death from any cause at a follow-up between 24 and 45 months? Simultaneously, will Rivaroxaban monotherapy be superior in regards to major bleeding?
Design: 1:1 open-label non-inferiority RCT, with blinded event adjudicators, performed in Japan. https://www.nejm.org/doi/full/10.1056/NEJMoa1904143
AFIRE trial summary
2,236 pts were randomized to receive the combination of Rivaroxaban 15/10 mg plus a single antiplatelet (ASA or P2Y12 inhibitors chosen by physician) versus monotherapy with Rivaroxaban 15/10 mg. The trial was stopped early due to an excess of total death in the combination arm at a median of treatment of 23 months. Rivaroxaban monotherapy was non-inferior for the primary efficacy end point (4.14% vs 5.75% pt/year, HR 0.72, CI 0.55-0.95, p<.001) and was superior for the primary safety end point, major ISTH bleeding (1.62% vs 2.76% pt/year, HR 0.59, CI 0.39-0.89; p= .01).
Comments and Methodological review
In spite of AFIRE reproduces the expected results in terms of excess of major bleeding without affecting ischemic/thromboembolic outcomes, I will revisit some methodological issues which may affect internal validity and therefore weakens its external validity.
Non-inferiority design, imprecision and inconsistency of efficacy primary outcomes
Rivaroxaban monotherapy was expected to be superior over the combination therapy in regards to major bleeding (safety primary end point): monotherapy 35 vs combination 58 events, HR 0.59, CI 0.39-0.89, p=0.01. Therefore, the investigators had a bet on demonstrating non-inferiority in the efficacy primary end point (composite of stroke, systemic embolism, MI, unstable angina requiring revascularization or death from any cause). Hypothetically, these results would indicate quitting aspirin as an attractive strategy to improve net clinical benefit. So, let us concentrate on efficacy…
Sample size determination (n= 2,200) was based on the occurrence of 219 efficacy primary end points, a number that seems to be “insufficient” to generate a solid evidence, and particularly troublesome if we try to understand results across the many components of the end point. For instance, as it was expected MI rate was higher -but not significantly- (13 vs 8, HR 1.60, CI 0.67–3.87) in the NOAC monotherapy compared with the combination therapy (NOAC + ASA). Surprisingly, other thrombotic events, such as ischemic stroke and unstable angina, were lower -also not significantly- in the NOAC monotherapy arm (21 vs 28 and 13 vs 18 events, respectively). How to understand similar physiopathology and opposite results? I guess that these outcomes behaviour should be assumed as random error and imprecision provoked by insufficient events number.
We may further add that the early stop for benefit at a median of 24 months prevented a complete follow-up and potentially avoided more end points, since 50% of patients did not reach the minimum planned follow-up at 24 months. Early stop for benefit usually represents a methodological weakness and a potential cause of overestimation of the effect size and increased probability of a larger difference due to random error or chance. AFIRE was stopped because an excess of total death in the combination arm (monotherapy 41 vs combination 73, HR 0.55, CI 0.38-0.81). Interestingly, in spite of an increased rate of major bleeding in the combination arm, the difference in total death was not associated to fatal bleeding.
By its nature, in non-inferiority designs definitions of H0 and HA are interchanged, therefore HA states that “there is no difference between both treatments”. So, type II error means that “there is a difference when the truth is that there is no difference” (misleading, isn´t it?). Thus, power is accepting that results are similar when they are really similar. As a consequence, the majority of non-inferiority trials are underpowered and confirm HA (non-inferiority of the new treatment). The farther we establish the distance of the non-inferiority margin (maximum allowable excess of events in the new treatment arm), the higher the probability of rejecting HA. In AFIRE the margin was 1.46; it means that we would accept up to 46% increased of relative risk with Rivaroxaban monotherapy, but it was not explained by the authors how did they determine this margin neither in the main paper nor in the design paper. This issue adds uncertainty.
Finally, the usual ITT (intention to treat) analysis performed in superiority trials to control prognostic factors at the start, benefits H0 rejection in non-inferiority trials. In AFIRE a “modified ITT” (applied to exclude some randomized patients in a justified manner) was performed, however, a “per protocol” analysis, which is more appropriate in this type of design, was also accordingly reported. Both analyses supported non-inferiority of NOAC monotherapy in the efficacy primary end point.
How to explain more MIs in one side and less ischemic stroke in the other beyond chance?
Open-label treatments: randomization controls confounders at the begining of a trial but does not prevent bias or differences during follow-up. That is why whenever is possible, everybody involved in the trial should be blinded to the treatment assignment. If not, event adjudicators must be blinded. Blinding avoids cointerventions and outcomes ascertainment. For instance, since investigators were informed about treatment assigment in AFIRE, they may have looked more intensively for MI in the Rivaroxaban monotherapy arm (without ASA) measuring troponin more frequently than in the combination arm every time a patient referred chest pain. Otherwise, they could have required brain CT scans more frequently in the combination arm because of being worried about ICH (intracranial hemorrhage). These actions may have biased towards a higher rate of MI in the monotherapy arm and higher rate of stroke in the combination arm. Unfortunately, in AFIRE trial it was not implemented placebo for the aspirin; the investigators did not explain the reason neither in the design nor in the main paper. Therefore, risk of bias increases and decreases the evidence quality and external validity.
Research in context
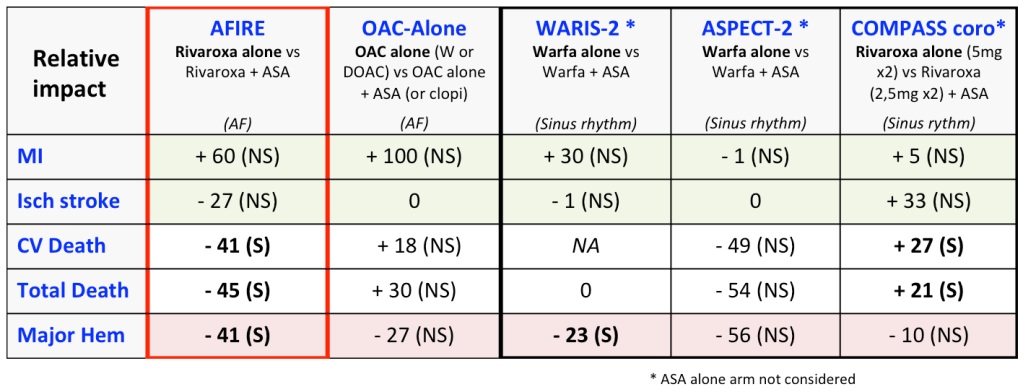
In this scenario, the aforementioned “general rule” is supported by results of RCTs performed in stable CAD in sinus rythm, such as ASPECT-2, WARIS-2 and COMPASS (coronary subgroup), performed before the AFIRE trial. The table shows the relative impact on death and major ischemic and bleeding events. OAC alone compared with OAC plus ASA shows a trend to benefit in major bleeding associated to a non-significant increase in ischemic outcomes (i.e., MI). We should consider that in COMPASS trial (subgroup of CAD patients without AF): 1) OAC alone was represented by a higher dose of Rivaroxaban, 2) each arm included >8,000 patients (more than the pool of all other RCTs), and 3) there was a significant increase in total and CV mortality in the OAC alone arm probably due to an increase of MI, stroke and coronary sudden death. Regarding RCTs in stable CAD patients with AF, there was only one prior inconclusive RCT (OAC-ALONE) prematurely terminated due to a slow recruitment. AFIRE results were already discussed.
I assume that striking differences in outcomes across trials derive from a significant clinical and statistical heterogeneity besides imprecision due to the a low sample size and other methodological issues in nearly all RCTs except COMPASS.
Take-home message
Oral anticoagulation with warfarin in patients with stable CAD and AF allows stopping ASA based on prior “indirect” randomized and observational evidence supported by clinical guidelines. AFIRE trial represents a great research effort and introduces new randomized evidence using a NOAC. Its results point towards the same direction of prior recommendations, however the methodological quality (or internal validity) deserves a “modest” qualification in my point of view. In the light of the stunning COMPASS results regarding mortality in patients in sinus rythm, I think we still need more solid data emerging from RCTs with NOACs in CAD and AF. Meanwhile, physicians can stop ASA intuitively based on the balance of thromboembolic/ischemic versus hemorrhagic risk, continuing with OAC alone following strictly dosing recommendations in case of NOACs.
